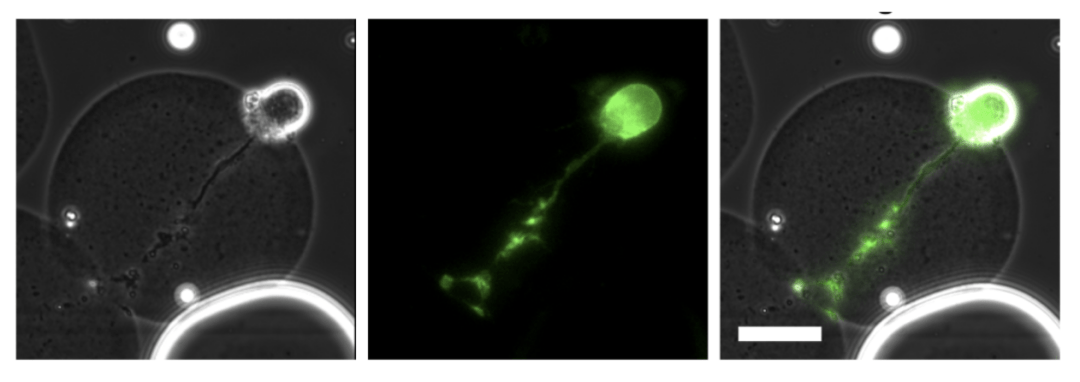
 A neuron grown in a micro gel infected with HSV-1 that expresses a yellow fluorescent protein. Image courtesy of Matthew Taylor.
A neuron grown in a micro gel infected with HSV-1 that expresses a yellow fluorescent protein. Image courtesy of Matthew Taylor.
BOZEMAN – A team of scientists from Montana State University recently published unique research examining how individual cells respond to viral infection. The work used state-of-the-art technology to culture cells and track infection in real time; it is the first known project to use microfluidic technology to culture, infect and track infection on a single-cell level.
Scientists from MSU’s College of Agriculture and Norm Asbjornson College of Engineering collaborated on the interdisciplinary work, which also involved MSU’s Center for Biofilm Engineering. The results of the project were published last week in Science Advances, one of the nation’s leading scientific journals, in a paper titled “Single-cell herpes simplex virus type 1 infection of neurons using drop-based microfluidics reveals heterogeneous replication kinetics.”
The faculty leaders on the project were Matthew Taylor, associate professor in the Department of Microbiology and Cell Biology, and Connie Chang, who spent nearly a decade in the Department of Chemical and Biological Engineering before taking a faculty position at the Mayo Clinic in Minnesota. Other members of the team included graduate students Jake Fredrikson, Luke Domanico and Shawna Pratt, as well as Emma Loveday, who finished her postdoctoral work while involved in the project and is now an assistant research professor in the MSU Center for Biofilm Engineering.
“It was truly a collaborative effort,” said Taylor. “The engineering principles and the technology behind it was all from Connie’s lab. Jake was just brilliant and had figured out how to grow neurons inside these little gels, basically on a micron scale. Each little bead grew a single cell."
Those gel beads were created using drop-based microfluidics, a process by which scientific experiments can be carried out on a microscopic scale more quickly and with less expense than through standard means. Chang likened the beads to tiny spheres of gelatin, made of a matrix that allows cells to grow just how they might in a petri dish, but with each individual cell in its own environment.
Fredrikson, who completed his Ph.D. in chemical and biochemical engineering in the spring of 2023, worked extensively on growing neurons – individual nerve cells – inside the tiny beads created through microfluidics. Once that process had been streamlined, the team introduced the cells to herpes simplex virus-1, a common virus that causes cold sores.
“It was basically a tiny tissue made in the lab, where we could infect it and watch virus infection happen in 3D and in real time,” said Chang. “Working with the Taylor lab was like the perfect blend; an engineer and a biologist working together to discover and do something totally new. This is the first time anyone has ever grown neurons at the single-cell level on this type of droplet."
The specific virus the team used had a uniquely engineered quality: It would fluoresce in different colors under a microscope, giving the team a visual trigger as infection progressed in the individual cells. When the virus infected the cell, it would appear yellow, and when it started replicating – the goal of viruses inside their host to perpetuate infection – it turned red. The cells were exposed to varying amounts of the virus to examine how they responded.
But not every cell responded the same way, Taylor said, which was unexpected. While most of the cells turned yellow, not all of them went on to turn red, meaning that some cells were effectively stopping the virus from replicating itself.
“The kind of remarkable thing is that every cell was exposed to an amount of virus that should produce infection,” said Taylor. “We know that the cells are infected because they’re yellow. Now we’re decoupling the process of infection from productive replication. We’re kicking the roots of virology, challenging these assumptions of what people think infection means and finding gaps between what we think is happening and what really is."
But how and why were some cells able to interrupt the viral replication process? That question will guide extensive future research, Taylor said.
“If cells can naturally shut down herpes viruses, and neurons can control it well, is there something that we can use to further limit productive replication? People have been trying to block herpes infection for eons, unsuccessfully,” he said. “But is there a way that we could shut down the virus and keep it from replicating?”
Further, said Chang, the implementation of microfluidics technology and the team’s first-of-its-kind examination at a single-cell level could create avenues for studying other types of cells, such as brain or lung cells, and examining the cellular response to other infections in search of treatments and cures.
Because drop-based microfluidics enables experimentation on such a small scale, it decreases the cost of research, widening access for scientists to conduct more cutting-edge research at less cost. The potential applications are endless, Taylor said.
“Microfluidics is very adaptable, and if you work with a very talented engineer like we did, they can design all sorts of architecture that can do different manipulations to the process,” he said. “What it really changes is that now you’re using much smaller quantities of everything. You can find rarer cell types, use less primary material and analyze larger quantities with less input.”
It took a collaboration across disciplines to precipitate such a novel accomplishment.
“These are always the best projects, the ones that bridge disciplines, where you’re merging your expertise with somebody else’s expertise,” said Chang. “Those are always the most high-impact, interesting and fun projects, and that’s why I’ve always loved this intersection of biology and engineering, bridging different disciplines.”
Taylor agreed, adding that the project wouldn’t have seen the success that it did without the blend of outstanding graduate students, inquisitive faculty and labs like the Center for Biofilm Engineering that coalesced at MSU.
“I’m most proud of the paper because it really demonstrates the heart of collaboration,” he said. “We just got the right match at the right time for it to be magic, and it really was magic. It was an amazingly productive collaboration.”






 A neuron grown in a micro gel infected with HSV-1 that expresses a yellow fluorescent protein. Image courtesy of Matthew Taylor.
A neuron grown in a micro gel infected with HSV-1 that expresses a yellow fluorescent protein. Image courtesy of Matthew Taylor.


News Comments
Thank you
Open Auditions for Annie
Monday, Sep. 16, 2024
I’m at the Bozeman airport where your painting, “Blowing East” is displayed. It’s absolutely gorgeous! Bravo, Marci!!
The Artists’ Gallery in Bozeman’s Emerson Cultural Center May Exhibits
Sunday, Jun. 30, 2024
This is so typical of a sign in, which we should not have to do to check if we or some one in our party got a permit. I have been working or "creating an account" for 30 minutes and just get the same ...
Smith River permit drawing results available
Sunday, Mar. 10, 2024
I have struggled with this podcast and my own participation therein, the event itself obviously traumatic, but beyond that my inability to reach anyone and convey anything resembling truth. The person ...
Billings, MT Case Becomes True Crime Podcast | 'An Absurd Result'
Marktokarski
Saturday, Jan. 20, 2024